-
PDF
- Split View
-
Views
-
Cite
Cite
Juan C Penagos Zuluaga, Simon A Queenborough, Liza S Comita, Flowering sex ratios and costs of reproduction in gynodioecious Ocotea oblonga (Lauraceae), Biological Journal of the Linnean Society, Volume 131, Issue 2, October 2020, Pages 344–355, https://doi.org/10.1093/biolinnean/blaa117
Close - Share Icon Share
Abstract
In gynodioecious plant species, both female and hermaphrodite individuals produce fruit, but only hermaphrodites produce pollen. Such sex-specific differences in reproductive investment may contribute to dimorphism, but the magnitude and ecological effects are still unclear, especially for gynodioecious tropical trees where collecting flowers and determining the sex is complex. We documented flowering and fruiting over three years in a natural population of Ocotea oblonga (Lauraceae) trees in a tropical moist forest, Panama. We determined sex from freshly collected flowers, counted and measured fruit, and used long-term growth data for each individual. We confirmed that O. oblonga is gynodioecious. No tree switched sex or had flowers of both sexes. The population was hermaphrodite-biased. We found no ecological differences in reproductive investment (seed, fruit, or tree size, or growth rate) between the sexes, indicating that the sex differential in the cost of reproduction is much smaller in woody gynodioecious taxa than in dioecious taxa. Females produced more fruit than hermaphrodites, which may contribute to their persistence in the population. Accordingly, and contrary to most studies of temperate gynodioecious populations, our study of a tropical tree shows no differential cost of reproduction in a hermaphrodite-biased population. Consequently, other factors such as seed fertility or herbivory could drive the biased sex ratio in this population.
INTRODUCTION
Plant species with dimorphic sexual systems such as dioecy or gynodioecy (where flowers of different sex are borne on separate individuals in a population) offer an opportunity to evaluate the costs of reproduction by examining how sexual differences in reproductive effort impact growth, survival and life-history traits (Vamosi et al., 2008). The ecological interactions between sexes also provide key information on the degree of divergence from hermaphroditism (where both sexes occur in the same plant). However, most information on dimorphic breeding systems comes from temperate and mostly herbaceous taxa (Caruso et al., 2016) and in the Neotropics studies of differential costs of reproduction have concentrated on dioecious species (Queenborough et al., 2007; Staudhammer et al., 2013; Riba-Hernández et al., 2016). In this study we confirmed gynodioecism in a tropical canopy tree species and investigated the short- and long-term cost of reproduction between female and hermaphrodite trees in a natural population in central Panama.
Gynodioecism is a dimorphic sexual system where female and hermaphroditic plants coexist within the same population (Darwin, 1877; Lloyd, 1974a). Gynodioecy has drawn special attention in evolutionary ecology for the low frequency of occurrence (2% of angiosperms genera; Dufay et al., 2014), but with multiple origins across the angiosperms. Although gynodioecism is not consistently related with dioecious taxa (Dufay et al., 2014), it is still considered an evolutionary pathway from hermaphroditism to dioecism. The majority of known gynodioecious species are temperate and herbaceous taxa (Dufay et al., 2014; Caruso et al., 2016), with few species described from the tropics (Caruso et al., 2016). This bias highlights our lack of understanding of the breeding systems of tropical trees, largely because of the difficulty of collecting flowers from multiple individuals of tall canopy and emergent tree species.
Females of gynodioecious taxa likely originate from a hermaphroditic ancestor through an interaction between nuclear and cytoplasmic genes and the abortion of pollen (i.e. they are male-sterile morphs) (Lewis, 1941; McCauley & Bailey, 2009). Hermaphrodites gain fitness through both pollen and seeds, whereas females gain fitness only through seeds fertilized by outcrossing with hermaphrodite individuals (Lloyd, 1974b). As a consequence, the proportion of individuals of each sex in a population reflects both the nature of the mutation (cytoplasmic/nuclear) and the balance between genetic drift and ecological constraints, such as competition for pollinators, seed dispersal and resistance to predation (Bawa, 1980; Delph, 1990b; Bailey & Delph, 2007).
Sex ratios in dimorphic populations reflect the effects of natural selection, gene flow, the environment and the fitness of each sex (Hamilton, 1967). In populations where both sexes are equally costly to produce or have equivalent fitness, a 1:1 sex ratio is expected (Fisher, 1930). However, male-biased sex ratios are frequently observed in many dioecious populations (Queenborough et al., 2007). This bias is largely due to the lower cost of producing pollen versus ovules and fruits (Charnov, 1982). In dioecious populations, the lower resource investment by males is often expressed as a smaller size at reproductive maturity, more frequent flowering, or higher growth and survival in males compared to females (Lloyd & Webb, 1977; Delph, 1999). In contrast, female flowers may have a smaller perianth (Delph et al., 1996). However, in gynodioecious populations, flowers on hermaphroditic individuals produce pollen and fruits, whereas flowers on female individuals produce only fruits with no investment in fertile pollen. This variation produces a smaller differential cost associated with reproduction in gynodioecious populations than in dioecious populations. Nevertheless, theoretical models suggest that females do have to compensate for their lack of pollen production (Lewis, 1941; Lloyd, 1975; Charlesworth & Charlesworth, 1978). Females may only persist in the population when their fitness is either slightly higher than (in the case of cytoplasmic sterility) or at least twofold (for nuclear sterility) that of hermaphrodites (Lewis, 1941). These fitness differences may be expressed as increased seed performance (Eckhart, 1992) or greater seedling and sapling vigour (Delph, 1990a; Thompson & Tarayre, 2000). Habitat quality also influences the relative fitness of females and hermaphrodites (Asikainen & Mutikainen, 2003; Chen et al., 2017) and higher frequencies of females are often correlated with poor soil quality (Ashman, 1999). Plant size also plays a role in sex expression, and smaller individuals in resource-limited locations may invest more in male flowers (Barrett, 1992).
Differences in floral traits such as the number of flowers, size, colour or rewards affect the visitation of pollinators. Studies in gynodioecious species of Geranium have found a higher frequency of visits of pollinators in hermaphroditic flowers (Asikainen, 2005; Van Etten & Chang, 2014), with a stronger negative effect on the relative fitness of females when they occur at low frequency (van Etten & Chang, 2014). In Lauraceae, floral dimorphism is not evident between the sexes, but in a few species, the male inflorescence tends to produce more flowers. Flowers secrete nectar from glands at the base of the filaments of the third androecial whorl, and in a few species, also from the head of the staminodes in the fourth whorl. The nectar is produced when the pollen is available (Rohwer, 2009), and likely attracts small insects (Kubitzki & Kurz, 1984).
Few tropical woody gynodioecious species have been reported (Renner, 2014; Caruso et al., 2016). However, the Lauraceae appears to be a good candidate for the occurrence of gynodioecism: Several Neotropical species have been described as possibly gynodioecious, and some taxa previously described as dioecious are now thought to be gynodioecious (Rohwer, 1986; Gibson & Diggle, 1998; van der Werff, 2014). Groups of related species in the Lauraceae are known to contain hermaphrodite, dioecious and gynodioecious taxa. However, identifying the correct breeding system from herbarium specimens is challenging, due to the presence of rudimentary structures in unisexual flowers and the difficulty of determining ovule and pollen fertility on dried specimens. In the species of Lauraceae described as dioecious, pistillate flowers produce staminodes (with slender-to-small filaments and flattened sterile anthers) that resemble regular stamens. In turn, staminate flowers can have a pistillode that in some cases is morphologically indistinct from a fertile gynoecium, preventing verification of ovule fertility, determination of whether a flower is male or hermaphrodite and, therefore, whether the species is dioecious or gynodioecious. Thus, many Lauraceae may have been mis-assigned to a breeding system. Careful dissection of fresh plant material is therefore required, along with direct field observations of flowering and fruiting.
In this study, we documented sex expression, reproduction and growth in a natural population of the likely gynodioecious tropical forest tree Ocotea oblonga (Meisn.) Mez in Panama over several reproductive episodes from 2016 to 2019. We asked the following questions: (1) Is Ocotea oblonga gynodioecious? (2) Is sex expression fixed over time? (3) Is the flowering sex ratio biased? (4) Are there signs of a cost differential? Specifically, are female trees smaller, have lower growth rates, or flower less frequently compared to hermaphrodite trees? and (5) Do female trees produce more or larger fruit than hermaphrodite trees?
Our study provides some of the first information on the reproductive ecology of a gynodioecious tropical tree species and opens the possibility for a better understanding of the intermediate steps between hermaphroditism and dioecism.
MATERIALS AND METHODS
STUDY SPECIES
Ocotea oblonga (Meisn.) Mez (Lauraceae) is a native Neotropical tree that grows up to 40 m tall. It occurs mostly in lowland forest, with few collections above 1500 m in the Andes, and is found from southern Mexico to north-eastern Bolivia and through the Guiana shield (http://tropicos.org/).
Ocotea oblonga has small trimerous flowers born in panicles on the most lateral twigs at the top of the crown. In central Panama, the species flowers during the rainy season, with trees blooming for 2–3 weeks between mid-June and late August (Croat, 1978). A shallow cupule subtends the one-seeded fruits and connects to a swollen cylindrical red pedicel.
The species was first described as dioecious (Mez, 1889), based on the dissection of only pistillate flowers. Prior to this study, O. oblonga was described as gynodioecious based on dried museum collections (Rohwer, 1986) but with no verification of ovule fertility on flowers that produced pollen (hermaphrodites).
STUDY SITE
We conducted this study in a natural population of O. oblonga in the tropical lowland seasonal moist forest on Barro Colorado Island (BCI) in central Panama. BCI is a 1567 ha island located in the Panama Canal, covered by undisturbed forest with areas of old secondary forest (Leigh, 1999). The island has a marked dry season from mid-December to late April or early May and a rainy season from May to December. Mean annual precipitation is ~2500 mm (Leigh, 1999).
We studied all the 350 mapped individuals of O. oblonga found in five long-term plots on BCI (50-ha, 25-ha, 10-ha and two 6-ha plots) with a total area of 97 ha. The 50-ha BCI forest dynamics plot (FDP) is located in predominantly old-growth forest on the plateau in the middle of BCI. It is a rectangular area of 1000 × 500 m wherein all trees ≥ 1 cm diameter at 1.3 m above ground (diameter breast height, DBH) were mapped, marked, identified and measured in 1981–1982 and then every 5 years starting in 1985 (Hubbell & Foster, 1992). Data on the DBH of each living stem in the FDP are available from censuses in 1982, 1985, 1990, 1995, 2000, 2005, 2010 and 2015. The FDP has relatively homogeneous topography and soil conditions with some variation in elevation and edaphic conditions (Harms et al., 2001; John et al., 2007). Within the FDP, O. oblonga (N = 321) is associated with slopes, which tend to be wetter than plateaus (Harms et al., 2001). Kriged estimates of soil nutrients for each 20 × 20 m quadrat within the plot are available (John et al., 2007). We also surveyed O. oblonga in four smaller long-term plots on BCI, wherein all trees ≥ 20 cm DBH have been mapped (N = 29). All the plots were located within ~1 km of the 50-ha FDP. Data on the DBH of trees in these plots were available from 2004 and 2014.
Each mapped tree was visited during the flowering (June–August) and fruiting seasons (February–March) from 2016 to 2019 (in the 50-ha FDP) or from 2017 to 2019 (in the smaller plots). Eight additional reproductive trees located outside the plots were encountered opportunistically (e.g. along trails) and were also monitored. In this study, the minimum observed reproductive size (DBHmin) was defined as the smallest DBH of a flowering tree recorded on the FDP. All trees with DBH ≥ DBHmin were categorized as adults and divided into two classes based on flowering status during this study: reproductive adult (trees with flowers) or non-reproductive adult trees (trees without flowers). Once a year for all trees of reproductive size, we measured DBH, height and light availability to the crown. To estimate light availability, we used the crown illumination index (CII; Clark & Clark, 1992, adapted from Dawkins & Field, 1978). Individuals were scored on along a simple ordinal scale of 1–5 [1, no direct light; 2, only lateral light; 3, some vertical light (10–90%); 4, full vertical illumination; and 5, crown fully exposed to vertical and lateral light].
SEX DETERMINATION, FLOWERING AND FRUITING
At each visit, trees were scored as flowering/non-flowering and fruiting/non-fruiting. Flowers and inflorescences were collected directly from the tree crown using a slingshot (Big Shot, SherrillTree, Greensboro, North Carolina), launching an 8-oz weight attached to a throwline (Petzl Airline) above the reproductive branches and up to 40 m high to shake flowers off or break the terminal branches < 1 cm diameter. Following collection, flowers were dissected by hand using a razor blade and mounted as longitudinal sections on semi-permanent slides in a 20% solution of calcium chlorohydrate (Keating, 2014). We examined the sections under a light microscope (Zeiss Axio Scope, A1, Jena, Germany), checking for pollen and/or developed ovaries in 10–20 flowers per tree and from multiple collections during each reproductive season.
To confirm the breeding system of O. oblonga (Q1) and check if trees varied in their sex expression (Q2), we checked for the presence of female and hermaphrodite trees. Here, we defined female trees by the absence of pollen in all mature flowers examined. Due to the presence of a sterile pistillode in the staminate flowers of dioecious Lauraceae, we defined hermaphrodite trees as those with flowers that produced pollen, and separated them as fruiting or non-fruiting hermaphrodites based on whether they produced fruits during the same reproductive season. We documented the production of fruits for each reproductive tree in the FDP from 2016 until 2019 and in the small plots from 2017 to 2019.
To estimate the number of mature fruits produced by each tree (Q5), we established four transects of 2 × 10 m under each tree, heading north, east, south and west from the base of the trunk. We collected and counted the number of mature fruits and seeds per transect each week for 8 weeks between late January and early April in 2017 and 2018. In 2017, we promptly weighed each fresh fruit and the single seed using a Gemini-20 Scale (0.001 g, American Weigh, Norcross, GA, USA). Fruits of O. oblonga are commonly attacked by insects, perforating the seed. Those fruits were excluded from the analyses of fruit and seed weight.
DATA ANALYSIS
To test for biased flowering sex ratios in each year and the total population sex ratio (Q3), we used the G test for goodness of fit (Queenborough et al., 2007). The sex ratio was calculated as: hermaphrodites / (hermaphrodites + females) (West, 2009), for each year separately. Because individuals were found to be consistent in their sex throughout the study, we also calculated total population sex ratio using data on the sex of all of the individuals that flowered in any year of the study and again tested for a biased total population sex ratio with a G test.
We tested for a cost of reproduction (Q4 and Q5) in three ways. First, to examine differences in size between the sexes, we compared the distribution of tree DBHs and heights between females and hermaphrodites using a Kolmogorov–Smirnov test. Second, we tested for differences in relative growth rate (RGR) as a function of sex (female versus hermaphrodite) for all trees in the plots. RGR was calculated as (ln(DBHt2) – ln(DBHt1)) / time in years, where t1 and t2 are time 1 and time 2, respectively (Paine et al., 2011). We calculated RGR for each tree in each census interval between 1982 and 2015 in the 50-ha FDP (see above), between 2004 and 2014 for individuals in the smaller plots and for the interval between the last plot census (2015 for the FDP, 2014 for smaller plots) and 2019 (our data). Ocotea oblonga often grow buttresses that may extend above 1.3 m, which may necessitate moving the point of measurement up the trunk. We therefore excluded measurements where DBH was measured at different heights for t1 and t2, or where data were not taken (we did not measure DBH for buttress trees in 2019).
RGR was modelled as a function of centred and scaled DBHt1 and tree sex (hermaphrodites, females and non-reproductive) using a linear mixed-effects model in the R package lme4 (Bates et al., 2015). To account for the repeated measurements of DBH over time, we included tree tag (tree identifier number) as a random effect. To account for the effects of annual variation in insolation and rainfall, we also included census interval as a random effect. We also tested for an effect of fruit production on RGR between 2015 and 2019. RGR from the last census interval (50-ha FDP: 2015–2019; and small plots: 2014–2019) was modelled as a function of centred and scaled DBHt1 and whether a tree had produced fruit during the study (N = 10, five hermaphrodite and five female) or not (N = 20, 17 hermaphrodite and three female) using linear regression.
In preliminary analyses, we explored whether growth models were improved, or the effect of sex on growth altered, by including information on light (CII; for growth between 2015 and 2019) or soil nutrients (for all census intervals in the 50-ha plot). We found no significant effects of these variables and so excluded them from the final models.
Third, we tested for differences in (1) number of fruit and (2) the weight of individual fruits and seeds produced between female and hermaphrodite trees (Q5). To test (1), we first ran a generalized linear model of whether a tree fruited or not (i.e. a binomial response) as a function of sex, tree DBHt1 (scaled and centred) and light availability (CII). Then for only trees that fruited in any year of the study we ran a generalized linear model of the total number of fruits produced during the whole time of the study as a function of sex, DBHt1 (scaled and centred) and light availability (CII), with a Poisson link to account for the count data. To test (2), we ran two linear mixed-effects models, one of fruit mass and one of seed mass, as a function of tree sex, with tree tag as a random effect to account for the multiple fruit samples per tree. All analyses were conducted in the software environment R 3.5.1 (R Core Team, 2018).
RESULTS
The minimum reproductive size of Ocotea oblonga observed over the 3 years of the study was 14.2 cm DBH. Of the 321 mapped O. oblonga trees ≥ 1 cm DBH in the 50-ha FDP, where all potentially reproductive individuals were visited, we encountered 36 adult trees ≥ 14.2 cm DBH. In other smaller plots we counted 29 trees ≥ 14.2 cm DBH, plus eight additional trees encountered outside of the plots. Of these 73 adult-size trees, we observed 64 trees that produced flowers at least once during the study, but only 22 trees that produced fruits at least once. Visual estimation of the inflorescences covering the crown varied from 40% to 90%. Flowers were rarely observed during the dry season, and then only very few (< 10% of the crown covered). Fruits matured from late February to early April and only two trees produced fruit outside of this season.
GYNODIOECY CONFIRMED FOR OCOTEA OBLONGA
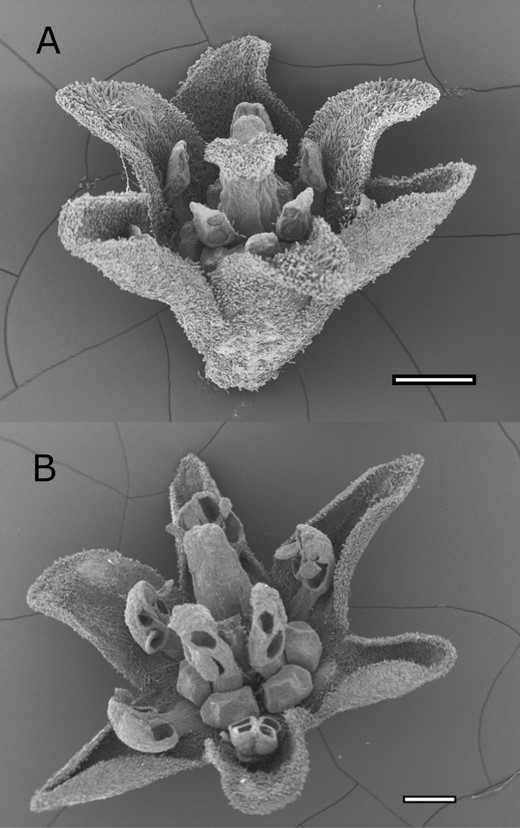
We examined flowers of all 64 reproductive trees and found that the population exhibited only two flower morphs: female and hermaphrodite. No other flower morphology was encountered. The female morph bore nine short staminodes with vestigial anthers (i.e. no pollen). This morph had a larger stigma that grew above the inner three staminodes (Fig. 1A). The hermaphrodite morph had all fertile stamens (i.e. with pollen) and a short stigma surrounded by the inner anthers (Fig. 1B). All flowers contained a gynoecium and there were no abnormalities that suggested female sterility. Both morphs expanded the tepals during anthesis. In female flowers the tepals were rarely expanded horizontally, whereas in hermaphrodite flowers the tepals were fully expanded horizontally.
Scanning electron microscope of flowers of Ocotea oblonga (Lauraceae) from Barro Colorado Island, Panama, collected in 2016. A, Female flower, showing undeveloped anthers and stigma larger than staminodes. B, Hermaphroditic flower, showing developed anthers and reduced stigma. Scale bar 0.5 mm.
From the multiple collections within and across reproductive seasons, each tree was observed to produce only one of the floral morphs, which are easy to differentiate: no tree was observed to switch sex between flowering episodes or to produce flowers of both sexes. Thus, protandry and protogyny are very unlikely to be confused with breeding system and we conclude that this population of O. oblonga is gynodioecious.
OCOTEA OBLONGA FLOWERING SEX RATIO IS HERMAPHRODITE-BIASED
Of the 64 individuals that flowered in at least one of the three years of the study, 19 were female and 45 were hermaphrodite (Table 1). Within the 50-ha FDP, we found a total of 29 reproductive trees (22 hermaphrodite and seven female). In any one year from 2016 to 2019, we documented flowers in 2–7 females and 13–21 hermaphrodites. During the same period, all female trees but only five hermaphrodites produced fruits at least once; the remaining 17 hermaphrodites flowered but did not produce fruits. In the small plots, we identified 27 reproductive trees (18 hermaphrodites and nine females). In any one year from 2017 to 2019, we documented flowers in 4–9 females and 9–16 hermaphrodites. During the two reproductive seasons, five female and one hermaphrodite produced fruits, and four female and 17 hermaphrodites flowered but did not produce fruits.
Flowering and cumulative sex ratios (proportion of females) for five populations of Ocotea oblonga on Barro Colorado Island, Panama. Ntot = total no. trees censused, reproductive and non-reproductive; Nfem: total no. of female trees; Nherm: total no. of hermaphroditic trees; Nrep = no. trees that flowered in a given year or set of years; Pherm = sex ratio (proportion of Nrep that are hermaphrodite). Significance denoted by asterisks: *P < 0.05; **P < 0.01; ***P < 0.001 (G test for random deviation of sex ratios from 1:1)
| . | . | . | . | 2016 . | 2017 . | 2018 . | Cumulative . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plot . | Ntot . | Nfem . | Nherm . | Pherm . | Nrep . | Nherm . | Pherm . | Nrep . | Nherm . | Pherm . | Nrep . | Nher . | Pherm . | Nrep . |
| 50 ha | 36 | 7 | 22 | 75.9** | 28 | 21 | 75.9** | 29 | 22 | 65.0* | 20 | 13 | 75.9** | 29 |
| Small plots | 29 | 9 | 18 | 66.7 | 24 | 16 | 60 | 10 | 6 | 66.7 | 27 | |||
| 25 ha | 4 | 1 | 2 | 66.7 | 3 | 2 | 66.7 | 3 | 2 | 66.6 | 3 | |||
| 10 ha | 5 | 2 | 2 | 66.7 | 3 | 2 | – | 1 | 0 | 50.0 | 4 | |||
| 6 ha Zetek | 19 | 6 | 13 | 66.7 | 18 | 12 | 60.0 | 5 | 3 | 68.0 | 19 | |||
| 6 ha Pearson | 1 | 0 | 1 | 0 | 0 | 0 | 100.0 | 1 | 1 | – | 1 | |||
| Outside plots | 8 | 3 | 5 | 50 | 2 | 1 | 57.1 | 7 | 4 | 50.0 | 6 | 3 | 62.5 | 8 |
| Population | 73 | 19 | 45 | 73.3*** | 30 | 22 | 70** | 60 | 42 | 61.1 | 36 | 22 | 70.31*** | 64 |
| . | . | . | . | 2016 . | 2017 . | 2018 . | Cumulative . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plot . | Ntot . | Nfem . | Nherm . | Pherm . | Nrep . | Nherm . | Pherm . | Nrep . | Nherm . | Pherm . | Nrep . | Nher . | Pherm . | Nrep . |
| 50 ha | 36 | 7 | 22 | 75.9** | 28 | 21 | 75.9** | 29 | 22 | 65.0* | 20 | 13 | 75.9** | 29 |
| Small plots | 29 | 9 | 18 | 66.7 | 24 | 16 | 60 | 10 | 6 | 66.7 | 27 | |||
| 25 ha | 4 | 1 | 2 | 66.7 | 3 | 2 | 66.7 | 3 | 2 | 66.6 | 3 | |||
| 10 ha | 5 | 2 | 2 | 66.7 | 3 | 2 | – | 1 | 0 | 50.0 | 4 | |||
| 6 ha Zetek | 19 | 6 | 13 | 66.7 | 18 | 12 | 60.0 | 5 | 3 | 68.0 | 19 | |||
| 6 ha Pearson | 1 | 0 | 1 | 0 | 0 | 0 | 100.0 | 1 | 1 | – | 1 | |||
| Outside plots | 8 | 3 | 5 | 50 | 2 | 1 | 57.1 | 7 | 4 | 50.0 | 6 | 3 | 62.5 | 8 |
| Population | 73 | 19 | 45 | 73.3*** | 30 | 22 | 70** | 60 | 42 | 61.1 | 36 | 22 | 70.31*** | 64 |
Flowering and cumulative sex ratios (proportion of females) for five populations of Ocotea oblonga on Barro Colorado Island, Panama. Ntot = total no. trees censused, reproductive and non-reproductive; Nfem: total no. of female trees; Nherm: total no. of hermaphroditic trees; Nrep = no. trees that flowered in a given year or set of years; Pherm = sex ratio (proportion of Nrep that are hermaphrodite). Significance denoted by asterisks: *P < 0.05; **P < 0.01; ***P < 0.001 (G test for random deviation of sex ratios from 1:1)
| . | . | . | . | 2016 . | 2017 . | 2018 . | Cumulative . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plot . | Ntot . | Nfem . | Nherm . | Pherm . | Nrep . | Nherm . | Pherm . | Nrep . | Nherm . | Pherm . | Nrep . | Nher . | Pherm . | Nrep . |
| 50 ha | 36 | 7 | 22 | 75.9** | 28 | 21 | 75.9** | 29 | 22 | 65.0* | 20 | 13 | 75.9** | 29 |
| Small plots | 29 | 9 | 18 | 66.7 | 24 | 16 | 60 | 10 | 6 | 66.7 | 27 | |||
| 25 ha | 4 | 1 | 2 | 66.7 | 3 | 2 | 66.7 | 3 | 2 | 66.6 | 3 | |||
| 10 ha | 5 | 2 | 2 | 66.7 | 3 | 2 | – | 1 | 0 | 50.0 | 4 | |||
| 6 ha Zetek | 19 | 6 | 13 | 66.7 | 18 | 12 | 60.0 | 5 | 3 | 68.0 | 19 | |||
| 6 ha Pearson | 1 | 0 | 1 | 0 | 0 | 0 | 100.0 | 1 | 1 | – | 1 | |||
| Outside plots | 8 | 3 | 5 | 50 | 2 | 1 | 57.1 | 7 | 4 | 50.0 | 6 | 3 | 62.5 | 8 |
| Population | 73 | 19 | 45 | 73.3*** | 30 | 22 | 70** | 60 | 42 | 61.1 | 36 | 22 | 70.31*** | 64 |
| . | . | . | . | 2016 . | 2017 . | 2018 . | Cumulative . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plot . | Ntot . | Nfem . | Nherm . | Pherm . | Nrep . | Nherm . | Pherm . | Nrep . | Nherm . | Pherm . | Nrep . | Nher . | Pherm . | Nrep . |
| 50 ha | 36 | 7 | 22 | 75.9** | 28 | 21 | 75.9** | 29 | 22 | 65.0* | 20 | 13 | 75.9** | 29 |
| Small plots | 29 | 9 | 18 | 66.7 | 24 | 16 | 60 | 10 | 6 | 66.7 | 27 | |||
| 25 ha | 4 | 1 | 2 | 66.7 | 3 | 2 | 66.7 | 3 | 2 | 66.6 | 3 | |||
| 10 ha | 5 | 2 | 2 | 66.7 | 3 | 2 | – | 1 | 0 | 50.0 | 4 | |||
| 6 ha Zetek | 19 | 6 | 13 | 66.7 | 18 | 12 | 60.0 | 5 | 3 | 68.0 | 19 | |||
| 6 ha Pearson | 1 | 0 | 1 | 0 | 0 | 0 | 100.0 | 1 | 1 | – | 1 | |||
| Outside plots | 8 | 3 | 5 | 50 | 2 | 1 | 57.1 | 7 | 4 | 50.0 | 6 | 3 | 62.5 | 8 |
| Population | 73 | 19 | 45 | 73.3*** | 30 | 22 | 70** | 60 | 42 | 61.1 | 36 | 22 | 70.31*** | 64 |
In every year and summing across all years, the whole population was significantly hermaphrodite-biased (Table 1). For individuals in the 50-ha FDP, the sex ratio was also significantly hermaphrodite-biased (4:1). Similarly, in the small plots the number of hermaphrodites always exceeded females, although the bias in the sex ratio was not statistically significantly (Table 1).
NO DIFFERENCE IN HEIGHT OR DBH BETWEEN FEMALE AND HERMAPHRODITE TREES
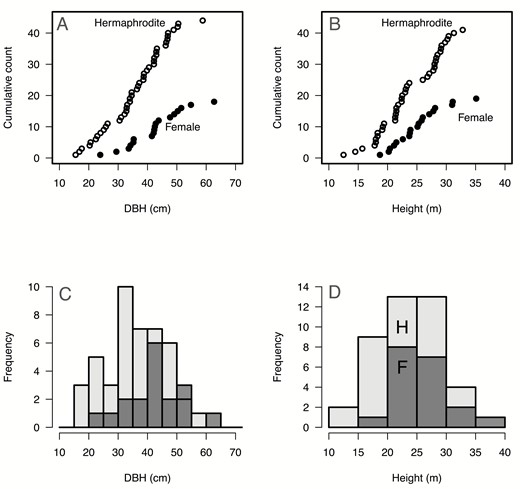
The minimum reproductive size we encountered (14.2 cm DBH) was a hermaphrodite tree, which was smaller than the minimum size of reproductive female tree encountered (23.9 cm). However, there was no significant difference in the distribution of DBH values between the two sexes (Kolmogorov–Smirnov test: D = 0.29785, P = 0.1897, Fig. 2A). Similarly, the minimum observed tree height in hermaphrodites (10.5 m) was shorter than the minimum height of female trees (18.1 m), but again no significant difference in the distribution of height values was detected (Kolmogorov–Smirnov test: D = 0.24519, P = 0.416, Fig. 2B).
Density distribution of hermaphrodite (H) and female (F) trees of Ocotea oblonga on Barro Colorado Island, Panama, in relation to tree size. There was no significant difference between sexes in: (A) diameter (DBH, in cm; Kolmogorov–Smirnov test: D = 0.32576, P = 0.1329), (B) height (m; Kolmogorov–Smirnov test: D = 0.24519, P = 0.416), (C) histogram of the DBH and (D) of the height. Female: light grey, hermaphrodite: dark grey.
NO DIFFERENCE IN GROWTH BETWEEN FEMALE AND HERMAPHRODITE TREES
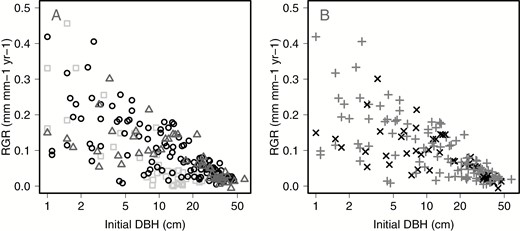
Contrary to our predictions, there was no significant difference in RGR between female and hermaphrodite trees in the 50-ha FDP between 1982 and 2015 and the small plots between 2004 and 2014 (Fig. 3A, Table 2). There was also no significant difference in RGR between trees that produced fruit or not in the most recent census interval (Fig. 3B, Table 2).
Linear mixed-effects model, linear model and generalized linear regression of relative growth rate (RGR), fruit weight and seed weight of sampled Ocotea oblonga trees on Barro Colorado Island (BCI), Panama, in 2016–2019. Model 1: linear mixed-effects model of RGR as a function of initial DBH and sex (baseline = female, H = hermaphrodite, NR = not reproductive; random effects: tree identifier and census interval). Model 2: Linear model of RGR as a function of production of fruits (Fruits, No-fruits) and DBH in the last census interval (50-ha FDP: 2015–2019, small plots: 2014–2019). Model 3: Generalized linear model of probability of fruiting (2016–2019), between female and hermaphrodite trees. Model 4: Generalized linear regression model for the differences in fruit production between female and hermaphrodite trees (2016–2019). Model 5: Linear mixed effects model for the differences in fruit weight between female and hermaphrodite (2016–2019). Model 6: Linear mixed effects model for the differences in seed weight between female and hermaphrodite (2016–2019). Significance denoted by asterisks: *P < 0.001
| . | Model 1 . | Model 2 . | Model 3 . | Model 4 . | Model 5 . | Model 6 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . |
| Intercept | 0.104 | 0.015 | 0.0815 * | 0.010 | 7.020 * | 0.046 | 7.020 * | 0.046 | 1.311 | 0.074 | 0.847 | 0.049 |
| Initial DBH | –0.061 | 0.006 | –0.032 | 0.007 | –0.617 * | 0.027 | –0.617 * | 0.027 | ||||
| Sex: H | 0.0007 | 0.015 | –2.526 * | 0.064 | –2.526 * | 0.064 | –0.016 | 0.150 | –0.113 | 0.098 | ||
| Sex: NR | –0.033 | 0.018 | ||||||||||
| Production of fruits | –0.004 | 0.012 | ||||||||||
| N | 205 | 30 | 62 | 21 | 230 | 235 | ||||||
| . | Model 1 . | Model 2 . | Model 3 . | Model 4 . | Model 5 . | Model 6 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . |
| Intercept | 0.104 | 0.015 | 0.0815 * | 0.010 | 7.020 * | 0.046 | 7.020 * | 0.046 | 1.311 | 0.074 | 0.847 | 0.049 |
| Initial DBH | –0.061 | 0.006 | –0.032 | 0.007 | –0.617 * | 0.027 | –0.617 * | 0.027 | ||||
| Sex: H | 0.0007 | 0.015 | –2.526 * | 0.064 | –2.526 * | 0.064 | –0.016 | 0.150 | –0.113 | 0.098 | ||
| Sex: NR | –0.033 | 0.018 | ||||||||||
| Production of fruits | –0.004 | 0.012 | ||||||||||
| N | 205 | 30 | 62 | 21 | 230 | 235 | ||||||
Linear mixed-effects model, linear model and generalized linear regression of relative growth rate (RGR), fruit weight and seed weight of sampled Ocotea oblonga trees on Barro Colorado Island (BCI), Panama, in 2016–2019. Model 1: linear mixed-effects model of RGR as a function of initial DBH and sex (baseline = female, H = hermaphrodite, NR = not reproductive; random effects: tree identifier and census interval). Model 2: Linear model of RGR as a function of production of fruits (Fruits, No-fruits) and DBH in the last census interval (50-ha FDP: 2015–2019, small plots: 2014–2019). Model 3: Generalized linear model of probability of fruiting (2016–2019), between female and hermaphrodite trees. Model 4: Generalized linear regression model for the differences in fruit production between female and hermaphrodite trees (2016–2019). Model 5: Linear mixed effects model for the differences in fruit weight between female and hermaphrodite (2016–2019). Model 6: Linear mixed effects model for the differences in seed weight between female and hermaphrodite (2016–2019). Significance denoted by asterisks: *P < 0.001
| . | Model 1 . | Model 2 . | Model 3 . | Model 4 . | Model 5 . | Model 6 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . |
| Intercept | 0.104 | 0.015 | 0.0815 * | 0.010 | 7.020 * | 0.046 | 7.020 * | 0.046 | 1.311 | 0.074 | 0.847 | 0.049 |
| Initial DBH | –0.061 | 0.006 | –0.032 | 0.007 | –0.617 * | 0.027 | –0.617 * | 0.027 | ||||
| Sex: H | 0.0007 | 0.015 | –2.526 * | 0.064 | –2.526 * | 0.064 | –0.016 | 0.150 | –0.113 | 0.098 | ||
| Sex: NR | –0.033 | 0.018 | ||||||||||
| Production of fruits | –0.004 | 0.012 | ||||||||||
| N | 205 | 30 | 62 | 21 | 230 | 235 | ||||||
| . | Model 1 . | Model 2 . | Model 3 . | Model 4 . | Model 5 . | Model 6 . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . | Estimate . | ± SE . |
| Intercept | 0.104 | 0.015 | 0.0815 * | 0.010 | 7.020 * | 0.046 | 7.020 * | 0.046 | 1.311 | 0.074 | 0.847 | 0.049 |
| Initial DBH | –0.061 | 0.006 | –0.032 | 0.007 | –0.617 * | 0.027 | –0.617 * | 0.027 | ||||
| Sex: H | 0.0007 | 0.015 | –2.526 * | 0.064 | –2.526 * | 0.064 | –0.016 | 0.150 | –0.113 | 0.098 | ||
| Sex: NR | –0.033 | 0.018 | ||||||||||
| Production of fruits | –0.004 | 0.012 | ||||||||||
| N | 205 | 30 | 62 | 21 | 230 | 235 | ||||||
Relative growth rate (RGR, mm mm–1 year–1) of Ocotea oblonga trees on Barro Colorado Island, Panama, since 1982 as a function of tree sex, initial DBH. A, There was no significant effect of tree sex on RGR (linear mixed-effects model, accounting for repeated measures and census interval; non-reproductive = light grey squares, female = mid-grey triangles, hermaphrodite = black circles). B, There was no effect of previous fruit production on RGR within reproductive trees (simple regression; few fruit = grey cross, many fruit = black ×). In both cases, RGR decreased with DBH (fitted lines not shown).
FEMALE TREES PRODUCED MORE FRUIT THAN HERMAPHRODITES
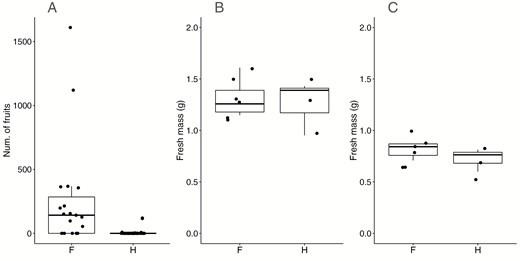
Across all years and all the reproductive trees, 15 of the 19 females and seven of the 45 hermaphrodites produced fruits during the study. Eight female trees and one hermaphrodite tree produced fruit in at least two consecutive seasons across all the plots. Female trees were significantly more likely to produce fruit than hermaphrodite trees (Table 2). Further, female trees produced a total of 54–1610 fruit over the three years, significantly more than hermaphrodite trees, which produced only 1–120 during the study (Fig. 4A, Table 2). In contrast to fruit number, there was no significant difference in fruit or seed mass between sexes (Fig. 4B, C, Table 2).
A comparison of number of fruits, fruit and seed mass between female (F; N = 13) and hermaphrodite (H; N = 9) Ocotea oblonga trees on Barro Colorado Island, Panama. A, Each point is the average number of fruits per tree during 2017 and 2018; number of fruits was significantly different between sexes. B, Fruit mass was not different between sexes; each point is the mean of the fresh mass of fruits for each tree. C, Seed fresh mass was not different between sexes; each point is the mean of the fresh mass of seed for each tree.
DISCUSSION
In a natural population of Ocotea oblonga located in the tropical moist forest on Barro Colorado Island, Panama, we assessed the reproductive status of 350 mapped O. oblonga trees in six plots, plus eight additional trees found along the trails. Through careful examination of flowers of the 64 trees that flowered during the study period, we were able to confirm the existence of the gynodioecious breeding system and document consistent hermaphrodite-biased sex ratios in this population. While female trees produced more fruit than hermaphrodite trees, there were no differences in tree size (DBH, height), growth (RGR), or fruit and seed weight between female and hermaphrodite trees.
CONFIRMATION OF GYNODIOECY IN A TROPICAL TREE
Our results indicate that the natural population of Ocotea oblonga is gynodioecious, with female and hermaphroditic trees confirmed from the dissection of fresh material and collection of fruits in the same season. Thus, our study corroborates observations from herbarium specimens of this species (Rohwer, 1986). Sexes exhibited conspicuous differences in floral morphology. Female flowers always had staminodes, with slender filaments and under-developed sterile anthers. No mature pollen was produced in any of the female flowers. Distinctive of this morphology was the large stigma, which protruded noticeably over the inner three staminodes. The tepals were ascendant and barely spread horizontally. In contrast, hermaphrodite flowers had fully developed and pollen-producing anthers. The stigma was narrow and surrounded by the inner stamens. The tepals completely spread on anthesis. No morphological evidence of female sterility was observed in these flowers. Differences in perianth size or flower number are often documented between the sexes in plant populations (Shykoff et al., 2003), but no obvious differences were found in this study. Further, despite the differences in the number of fruits produced—hermaphrodites of gynodioecious perennials may fruit irregularly with wide variation between seasons (Darwin, 1877)—we did not document any morphological differences between fruiting and non-fruiting hermaphrodite flowers.
Sexual dimorphism is common in Lauraceae, where dioecism has evolved independently multiple times (Chanderbali et al., 2001) often led by subtle morphological differentiation between sexes. Dimorphism is described for Ocotea tenera Mez & Donn. Sm, a small tree endemic to Costa Rica (Burger & van der Werff, 1990), which has been ambiguously described as subdioecious (Donnell Smith, 1903), gynodioecious (Gibson & Wheelwright, 1996; Gibson & Diggle, 1997, 1998) and dioecious (Wheelwright, 1992; Gibson & Diggle, 1997; Wheelwright et al., 2012). In Lauraceae, gynodioecism is suggested in dioecious species currently included in the O. smithiana and O. cernua group, especially in those with a well-developed pistillode in the flowers that produce pollen (van der Werff, 2017). Ocotea oblonga was described with a similar pistillode and verification of fruit production in the pollen-producing flowers demonstrated its fertility, suggesting that gynodioecy is also likely to occur in species of these two groups. Future ecological and taxonomical studies should carefully monitor natural populations of these Ocotea taxa and dissect floral material from multiple individuals to confirm the breeding system.
SEX EXPRESSION IS FIXED OVER TIME
Over three consecutive seasons, we found no change in the sex of any tree, nor did any tree have flowers of both sexes. Three reproductive episodes are few in the life of a tree and O. oblonga may switch or produce flowers of a different sex over longer timescales. However, the studied population was composed of trees of different ages and sizes, increasing the possibility of observing any such changes. Sex-switching has been reported from a few trees of O. tenera during a long-term study (Wheelwright, 1992). However, these switches were not correlated with age or spatial distribution.
Some cases of young trees switching from male to female with age have been found in other species, but it is not common. For example, in the dioecious Lindera benzoin (L.) Blume (Lauraceae) less than 2% of the plants switched (Primack, 1985). Similarly, small trees of Iryanthera juruensis Warb. (Myristicaceae) in Ecuador produce ramiflorous male flowers, but larger trees produce additional cauliflorous female flowers (Queenborough et al., 2007). In our study, however, we found no relationship between tree size and sex, suggesting that size-related switching does not occur in this population.
BIASED SEX RATIOS
The reproductive trees in our study were predominantly hermaphrodite (70%), and the population had a significantly hermaphrodite-biased sex ratio. This result differs from previous studies of tropical Lauraceae, where O. tenera was reported to have an unbiased sex ratio (Wheelwright, 1992). However, in the temperate dioecious relatives of Lindera, sex ratio varies from extremely biased to relatively unbiased. Lindera benzoin (L.) Blume has been reported to have a continual female bias over a period of 17 years (Cipollini et al., 2013), but across populations the sex ratio is relatively unbiased (Primack, 1985; Niesenbaum, 1992). In contrast, populations of Lindera subcoriacea Wofford are slightly male-biased (Wall et al., 2013), and in Lindera melissifolia (Walter) Blume populations are exceptionally male-biased, with 86% male individuals (Wright, 1994).
In gynodioecious species, sex ratio is regulated by the relative fecundity (seed/pollen) of each sex, the genetic inheritance of male sterility and environmental factors affecting longevity and mortality (Webb, 1999; Bailey & Delph, 2007). Female-biased populations have been correlated with higher seed fertility (Shykoff et al., 2003; Bailey & Delph, 2007) and poor soil nutrients in Sagittaria latifolia Willd (Alismataceae) (Yakimowski & Barrett, 2014) and Fragaria virginiana Mill (Rosaceae) (Ashman, 1999). Our populations did not show any differences in fruit or seed size between sexes or an effect of soil nutrients on growth. Instead, the higher frequency of hermaphrodites may be the result of differential mortality of adult individuals or another selective mechanism not tested in this study. The persistence of females could be related to higher fruit production, higher germination rates, or plant–enemy interactions of female seedlings, regardless of the origin of sterility. Future work should assess whether there is a biased sex ratio among different populations and if there is a differential survival of the now known-sex individuals of O. oblonga.
COST OF REPRODUCTION
In gynodioecious plants, hermaphrodites allocate resources to both pollen and ovules/fruits, whereas females do not invest in male structures. This difference in resource allocation has implications for performance and life-history strategy (Charnov, 1982). We found several hermaphrodite trees that flowered at a smaller size than the smallest female. We also found that although female trees produced more fruit than hermaphrodites, this greater cost borne by the females was not reflected in lower growth or in smaller fruits or seeds compared to hermaphrodites. Similar results were reported for species of Myristicaceae, with no significant sex effects on annual diameter growth (Queenborough et al., 2007). Other factors may offset these costs, such as the stored resources available to reproductive trees or access to new resources such as light. Light availability affects fruit production in many tropical canopy tree species (Graham et al., 2003).
Many tropical trees are pollen limited (Ghazoul, 2005). Dioecy is an obligate outcrossing mechanism inherently prohibiting self-pollination. In contrast, hermaphrodites can potentially self-pollinate and self-compatibility is common in gynodioecious species (Dufay & Billard, 2011). Many tropical plants, however, tend to have high rates of self-incompatibility (Larson & Barrett, 2000). In Lauraceae, cross-pollination is reported in hermaphrodite species, based on pollination experiments (Kubitzki & Kurz, 1984) or using population outcrossing (Gibson & Wheelwright, 1996). Additionally, flowers of Lauraceae are commonly visited by small Meliponinae (Kubitzki & Kurz 1984, and personal observation), which usually move among neighbouring trees (Gibson & Wheelwright, 1996). Even if O. oblonga is self-compatible, pollination and consequent fruit production is mediated by pollinators. Future studies of gynodioecious Ocotea should examine differences in pollinator visitation, pollination success and the effects of flowering neighbours on fruit production (e.g. Jones & Comita, 2008).
CONCLUSIONS
This study demonstrated the presence of gynodioecy in a hermaphrodite-biased population of Ocotea oblonga in Panama, but found little evidence of differences in patterns of resource allocation between the two sexes. The lack of a difference in long-term growth rates between female and hermaphrodite trees suggests that females compensate for their greater fruit production in other ways, highlighting the importance of using long-term data to monitor variation in sex ratios and resource allocation in tropical trees.
ACKNOWLEDGEMENTS
The authors are grateful to Joe Wright, Rick Condit and Stephen Hubbell for access to FDP data and permission to work within the plots. We are especially grateful to the forest crew and BCI staff who contributed to collecting the data and facilitating research at the site. Funding was provided by the Yale Tropical Resources Institute and the Yale Institute for Biospheric Studies. We thank the Missouri Botanical Garden for facilitating the use of the scanning electron microscope. We thank two anonymous reviewers for their helpful comments. J.C.P.Z. designed the project, collected and analysed data, prepared figures and wrote the manuscript. S.A.Q. analysed data, prepared figures, and revised the manuscript. L.S.C. designed the project, analysed data and revised the manuscript. All authors declare that they have no competing interests.
REFERENCES